Current Research
Our group studies pancreas developmental biology in several models, including fruit flies, mice, pigs, cultured stem cell lines, and with primary human tissues. We have innovated methods for studying pancreas biology in these models, and discovered conserved cellular, molecular and genetic mechanisms governing islet cell growth, development and function. Based on our findings, we have active clinical collaborations to investigate translational potential of our work. One goal of our work is to translate our studies into novel diagnostic and therapeutic strategies for common pancreatic disease states in humans, particularly diabetes mellitus and pancreatic cancer. I have also contributed to science education in multiple ways over the past 25 years as a PI and mentor in several graduate and post-graduate training programs at Stanford and a high school science program called Stan-X that now serves nearly 20 partner schools worldwide. We are committed to training the next generation of biomedical researchers.
DECONSTRUCTING AND RECONSTITUTING PANCREAS AND ISLET DEVELOPMENT
Deficiency of functional insulin-producing islet β-cells and dysfunction of glucagon-producing α-cells underlies the pathogenesis of diabetes mellitus, a disease with devastating autoimmune and pandemic forms. However, islet replacement in diabetes is ultimately limited by our inadequate understanding of mechanisms controlling islet formation and growth. To meet this challenge, we have been devoted to understanding the molecular, genetic, signaling and cellular basis of pancreatic development in mice, pigs and humans.
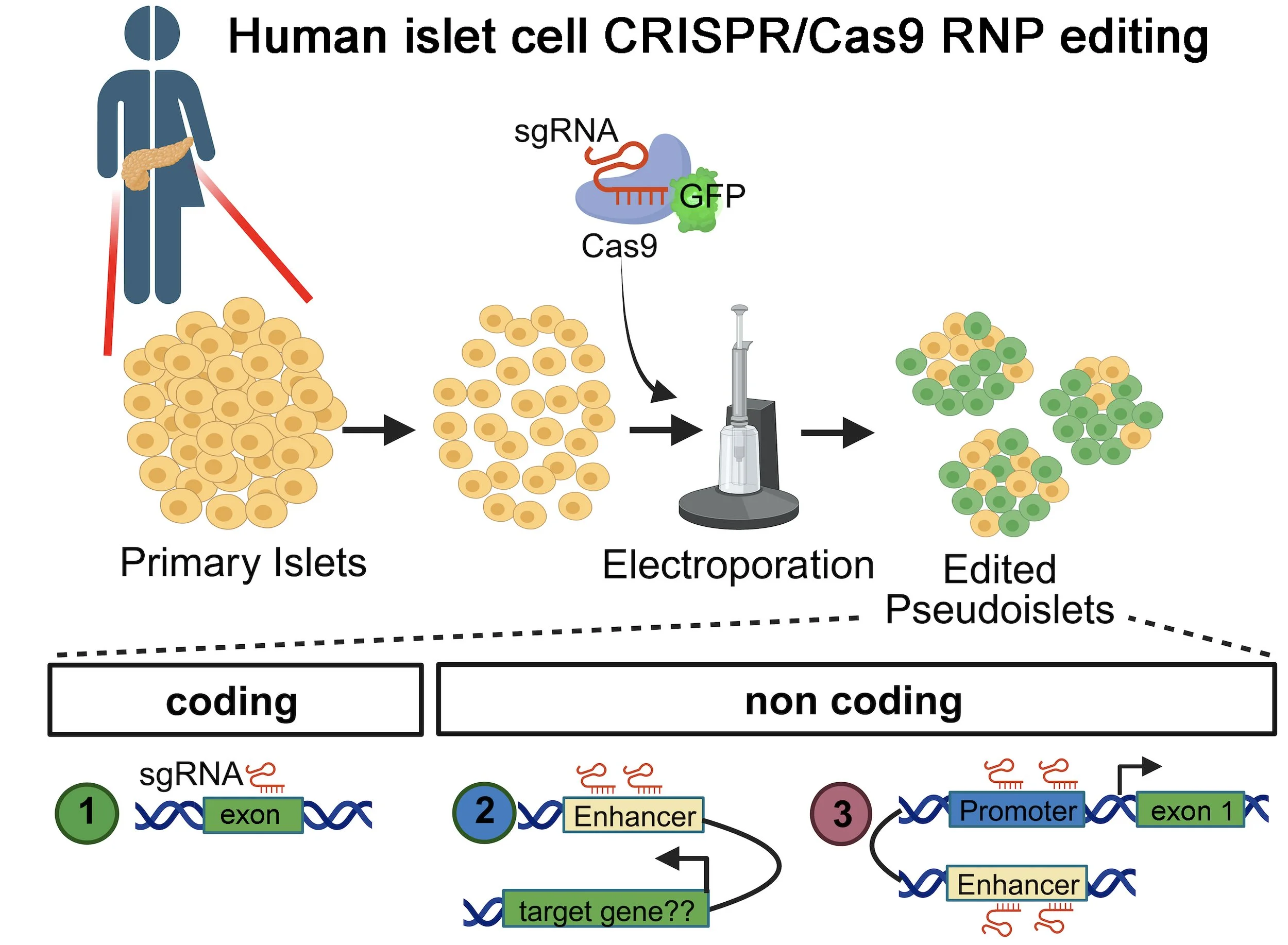
We have discovered mechanisms governing mouse and human pancreatic cell growth, differentiation and fate choices (Heit et al 2006; Goodyer et al 2012; Chen et al 2009, 2011; Arda et al 2016, 2018; Chakravarthy et al 2017; Bevacqua et al 2021a,b, 2023). This includes delineation of age-dependent transcriptome and chromatin changes in human islets using single cell and bulk RNA-Seq and ATAC-Seq (Arda et al 2016, 2018; Enge et al 2017), powerful imaging methods like CLARITY, and islet cell functional assessment with patch clamping (Camunas-Soler et al 2019; Dai et al 2022), CRISPR-Cas9 gene targeting (Bevacqua et al 2021b) and electroporation RNP CRISPR in primary human islet cells (Bevacqua et al 2023). We have also developed the pig as an alternative paradigm for studying pancreas and islet development (Kim et al 2020). These studies have revealed novel regulatory roles for factors like Calcineurin, NFAT, BCL11a, SIX2, SIX3, HNF1a and RFX6, in human islet cell development or maturation.
DIABETES AND HUMAN ISLET GENETICS
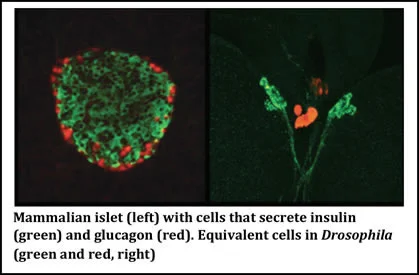
We discovered Drosophila endocrine cells that secrete insulin in collaboration with Stanford colleagues, and showed that this circulating hormone is crucial for regulation of fly growth and glucose homeostasis (Rulifson et al 2002). We then demonstrated that Drosophila cells secreting a glucagon-like hormone called AKH are also essential for glucose regulation. We showed that glucose-sensing and responses by AKH-secreting cells are governed by KATP channels, which also regulate stimulus-secretion coupling in mammalian islets (Kim and Rulifson et al 2004). We have also generated methods to measure circulating bioactive insulin in fruit flies (Park et al 2014). These discoveries have created new opportunities to use the powerful experimental advantages of Drosophila for genetic, developmental and lineage studies of ancestral islet-like cells to identify new regulators of pancreatic islet development, growth and function (Alfa et al 2015; Alfa and Kim, 2016; Park et al 2014; Peiris et al 2018) and insulin resistance (Tsao et al 2022).
Based on this remarkably conserved regulation of metabolism by hormones, we postulated that in vivo studies in Drosophila combined with appropriate human islet studies could elucidate fundamental aspects of diabetes genetics, including the mechanisms underlying polygenic risk. For example, we screened fly orthologues of dozens of genes linked by genome wide association studies (GWAS) to diabetes risk in humans, and identified novel fly regulators of insulin production and secretion (Park et al 2014; Peiris et al 2018). Using purified human islets, genetic approaches we have pioneered (Arda et al 2016), we then revealed that loss of the cognate human gene in human islets led to identical changes of insulin production and secretion (Peiris et al 2018; Bevacqua et al 2021a, b).
To foster the development of human islet genetic studies, we have pioneered gain- and loss-of-function approaches in primary human islets using 'pseudoislets' (reviewed in Friedlander et al 2021). This involves dispersion of primary islets into single cell suspensions followed by reaggregation. The transient dispersed state of islet cells permits lentiviral targeting approaches (Arda et al 2016; Peiris et al 2018; Bevacqua et al 2021a) and electroporation-based CRISPR RNP targeting (Bevacqua et al 2023). Reaggregation of islets into clusters then permits further studies, including insulin or glucagon secretion, electrophysiology including patch clamping, and molecular analysis (Dai et al 2022; Bevacqua et al 2021a,b; Qian et al 2023; Coykendall et al 2023). This strategy has opened a huge range of experimental approaches to study primary islets and stem cell progeny.
ACHIEVING ALLO-TOLERANCE AND SELF-TOLERANCE OF TRANSPLANTED ISLETS
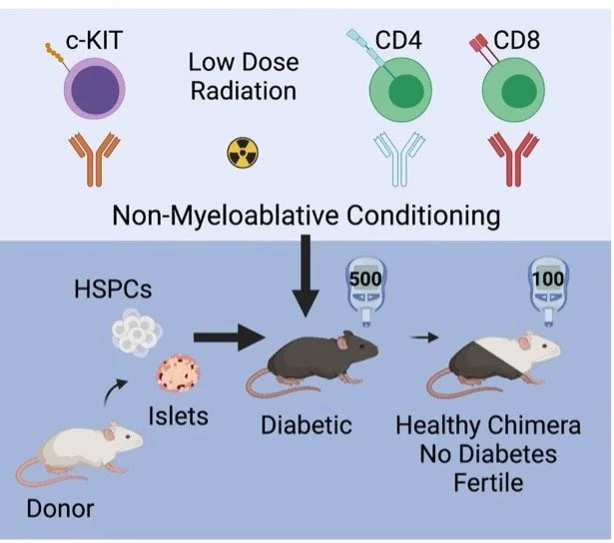
Transplantation of pancreatic islets from immune incompatible allogeneic donors can be curative after beta cell loss - like in type 1 diabetes - but requires toxic life-long immunosuppression (Bartlett et al 2016, Hering et al 2016). Cell therapies focused on promoting immunological tolerance rather than crude immune suppression are crucial to expand islet transplantation for use in a broader patient population (Leventhal and Mathew 2020). With our collaborators Drs. Judy Shizuru and Everett Meyer, we have explored methods to achieve both tolerance of allogeneic islets (allo-tolerance) and self-tolerance of islets in autoimmunity. This is possible with simultaneous donor-matched bone marrow transplantation to produce mixed chimerism, where donor and host hematopoietic stem cells co-exist and repopulate a tolerant immune system composed of both donor and host immune cells (Zuber and Sykes 2017, Pathak and Meyer 2020).
To create mixed hematopoietic chimerism, we are “conditioning” the host with reagents that are less toxic and more specific than standard radiation or chemotherapy. Thus, our work focused on innovating and optimizing bone marrow conditioning regimens to reduce toxicity and allow durable mixed chimerism in diabetic mice has promoted allogeneic islet transplantation and diabetes reversal. For example, using a mouse model of inducible diabetes developed in our lab (Bhagchandani & Chang et al 2022), we have shown long-term stable mixed chimerism after low toxicity conditioning and subsequent tolerance of fully mismatched allogeneic islets in non-diabetic and diabetic settings (Chang & Bhagchandani et al 2022). We see full protection of islets despite preserved immunocompetence to reject foreign islets, and the procedure is curative in diabetic mice without chronic systemic immunosuppression. These findings have motivated us to investigate and optimize this combination cell therapy in mouse models of autoimmune diabetes, mainly the non-obese diabetic (NOD) mouse, and to investigate both central and peripheral mechanisms of tolerance induction.
We are also engaged in developing systems like mouse models with 'humanized' hematopoietic and immune systems to discover conditioning regimens suitable for human populations. We are actively investigating the addition of reagents with promise for human translational trials with our clinical colleagues. These investigations are enabled by our development of clinical islet transplantation through the Stanford Pancreatic Islet Replacement and Immune Tolerance (SPIRIT) programs.
COLLABORATIONS
The Kim group has a long history of productive collaborations with the best scientists in the world, built on foundations of trust and respect. These interactions stem from association with individual investigators at Stanford and off-site, and Kim's leadership or participation in several multi-institutional or multi-investigator efforts around pancreas, islet, diabetes, pancreatic cancer, chronic pancreatitis, diabetes immunology, stem cells, and developmental biology. These include directing the P30-funded Stanford Diabetes Research Center (SDRC) since its inception in 2016, and participation on projects supported by the prior NIH Beta Cell Biology Consortium (BCBC), the current NIH Human Islet Research Network (HIRN), the Accelerating Medicines Partnership (AMP-T2D), the NCI/NIH Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC) consortium, and co-directing the Northern California JDRF Center of Excellence.
Within Stanford, this has resulted in widely-heralded publications related to pancreas, diabetes and hormonal research with leading investigators like Roel Nusse, Matthew Scott, Howard Chang, Gerald Crabtree, Karl Deisseroth, Steve Quake, Everett Meyer, Monte Winslow, Laura Attardi, Peter Jackson, Will Greenleaf, Judith Shizuru, Anna Gloyn, and others.
The Kim group has also had highly productive collaborations and publications with investigators outside Stanford, including Matthis Hebrok, Alex Marson, Anil Bhushan, Michael German, Hail Kim, Alvin Powers, Roland Stein, Christopher Wright, Matthew Meyerson, Rita Bottino, Markus Grompe, Patrick MacDonald, Pedro Herrera, Dale Greiner, Lenny Shultz, Ralph Hruban, Leif Groop, Roland Eils, Jorge Ferrer, and others.
INNOVATING SCIENCE EDUCATION
Science is principally about discovery. However, traditional secondary school and undergraduate science classes do not typically allow students to make actual discoveries, potentially limiting instruction and distorting an understanding of science. In the K-12 period, science is often taught by instructors without training in experimental design, execution or interpretation. This model leads to modes of science teaching that are unlinked to open-ended experimentation and the excitement of exploration and discovery. Instead, current science teaching often involves cycles of memorization and recall that paradoxically crush curiosity and wonder about the natural world. By contrast, in virtually all other subjects or areas, like English, Math, History, Music, Theatre Arts, Modern Languages, Athletics – innovative work has reduced or eliminated the ‘distance’ between the student and the primary text, material or experience.
In 2011, Stanford scientists and faculty at the Phillips Exeter Academy partnered to engineer a science teaching models based on experimental biology. A new science course (Bio 670) enabled Exeter students in 11th or 12th grade to perform open-ended research using the fruit fly, which has advantages of experimental and pedagogical simplicity, flexible scheduling, and cost feasibility. This course opened the exciting possibility of making novel scientific discoveries, and generating data and resources that can be published and used by practicing scientists.
From 2016 to 2023, additional collaborative efforts led to creation of 18 more programs, including at a high needs public school (the Pritzker College Prep School, Chicago), the Lawrenceville School in New Jersey, the Lowell High School (San Francisco), the Latin School (Chicago), the Chapin School (New York), and at Oxford University (U.K.). Collectively, this growing network of partners, including three public secondary schools, 14 independent secondary schools, and two universities, comprises the Stan-X programs. The output from the Stan-X courses at these schools has been published in five peer-reviewed articles (Kockel et al 2016; Kockel et al 2019; Chang et al 2022; Kim et al 2023; Rankin et al 2023).
Stan-X is currently in discussions with additional partner schools in the U.S., Asia and Europe, to develop collaborative experimental science teaching programs. These include urban public and independent high schools, several serving an underrepresented student population. To accommodate this growing global interest in the Stan-X concept, we developed a teaching academy called Discovery Now, in the summer of 2019. This training program was led by members of Stan-X, including Kim and instructors and undergraduate or doctoral students from Stanford, secondary students from Exeter, Lawrenceville, and invited Stan-X teachers.
- Research Highlight: 'Ultimately about discovery': High-school students experience hands-on biology research. Scope-Stanford Medicine.
- Research Highlight: Synergy and Science. Exeter Bulletin Fall 2016.
- Research Highlight: Farming Fruit Flies for Science